Kintor Pharmaceutical Limited (Kintor Pharma, HKG: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today announced its 2021 interim results and released an update on its recent progress in clinical development, commercialization (including license-out and production capacity expansion), and activities in Hong Kong capital market.
Dr.Youzhi Tong, Founder, Chairman, and CEO of Kintor Pharma, commented,
“In the first half of 2021, Kintor Pharma has made great progress in areas including research, clinical operations, and business cooperation with reputable partners. More specifically, proxalutamide, an innovative androgen receptor antagonist for the treatment of COVID-19 infection, is in three phase III multi-regional clinical trials (MRCT) approved by regulators in the United States, Brazil, and other countries. Recently, in a major milestone, proxalutamide was granted its first emergency use authorization (EUA) by the Paraguay. Looking further out, our company is accelerating the global clinical development of existing products pipeline and remains focused on creating value at the clinical stage, while pursuing new treatment for patients with unmet needs. At the same time, we continue to strive to create value for shareholders.”
First EUA Granted for Proxalutamide for Treating COVID-19 Inpatients
Proxalutamide is a nonsteroidal antiandrogen – specifically, a selective high-affinity silent antagonist of the androgen receptor (AR). The compound has a dual mechanism of action: It inhibits the androgen receptor competitively and decreases the expression of AR, effectively lowering the expression of the proteins ACE2 and TMPRSS2, which the novel coronavirus uses to invade host cells. Thus, proxalutamide prevents the coronavirus from infecting normal host cells, prevents viral replication and reproduction, and appears to treat novel coronavirus infections effectively. In addition, proxalutamide also promotes the clearance of pathogens and decreases inflammation by activating the Nrf2 pathway, which activates several antioxidative genes and proteins and reduces the intensity of the cytokine response, which is of clinical benefit to the most seriously ill COVID-19 patients.
Kintor Pharma has been studying the use of proxalutamide for the treatment of COVID-19 infection since early 2020, at the beginning of the COVID-19 pandemic. So far, the in vitro studies in the P3 laboratory have demonstrated that proxalutamide can effectively inhibit infections caused by the Alpha and Delta variants. The outcome of genome sequencing on COVID-19 inpatients in Brazil has shown that proxalutamide has effectively treated inpatients infected by Gamma variant.
In 2021, Kintor Pharma received greenlight from the U.S. Food and Drug Administration (FDA) and the approval from Brazilian Health Regulatory Agency (ANVISA) to conduct phase III clinical trials with proxalutamide in patients with COVID-19. The company is conducting two phase III MRCT of proxalutamide for the treatment of COVID-19 outpatients, and one phase III MRCT for COVID-19 inpatients in countries and regions including the United States, South America (including Brazil), the Europe, and Asia.
Most recently, the Ministry of Public Health and Social Welfare (MSPBS) of Paraguay granted Kintor Pharma an EUA for proxalutamide to treat hospitalized patients with COVID-19.
Pyrilutamide and GT20029 for Hair Loss and Acne
Androgenic alopecia (AGA) is a common form of hair loss in both men and women. Acne vulgaris is common in teenagers and young adults. Both AGA and acne affect people worldwide. Kintor Pharma has made progress in developing products for these two conditions.
Kintor Pharma’s pyrilutamide is a topical AR antagonist with a specific target that can inhibit the combination of AR and androgen in hair follicle sebaceous glands, treating both AGA and acne.
Pyrilutamide is undergoing phase II clinical trials in China for AGA. As of this writing, the trial data is being analyzed, and we expect to announce results soon. In April 2021, the first batch patients of pyrilutamide’s phase I/II clinical trial in China for acne vulgaris were enrolled and successfully dosed. Separately, the FDA approved pyrilutamide’s US-based phase II clinical trial for AGA in July 2021.
Kintor Pharma developed protein degradation chimera (PROTAC) compounds. The first, GT20029, can effectively block the AR pathway and physiological function by degrading the AR protein.
In April and July 2021, Kintor Pharma received clearance from the CDE and FDA to conduct phase I clinical trials with GT20029 for the treatment in AGA and acne in China and U.S.
In July, Kintor Pharma announced that the first subjects had been enrolled and dosed in a phase I clinical trial of GT20029 for the treatment in AGA and acne in China. GT20029 is the world’s first topical AR-PROTAC compound to enter the clinical stage.
We look forward to reporting progress on pyrilutamide and GT20029 soon.
Exploring Innovative Combination Therapies for Multiple Solid Tumor Types
In February 2018, Kintor Pharma obtained from Pfizer Inc. an exclusive license for GT90001, a fully humanized monoclonal, potential first-in-class antibody that inhibits ALK-1/TGF-beta signal transduction and tumor angiogenesis. GT90001 has the potential as a treatment in multiple solid tumor types.
GT90001 is now in phase II clinical trials in Taiwan (China) as a second-line combination therapy with Nivolumab, a PD-1 inhibitor, for metastatic hepatocellular carcinoma (HCC). The preliminary data from the ongoing Taiwan (China) phase II trial was released at ASCO GI 2021 and showed positive efficacy and safety results. Eight of 20 assessable patients had partial remission (PR), with an objective remission rate of 40 percent.
In February 2021, the U.S. FDA granted Kintor Pharma an investigational new drug (IND) application of GT90001 for a multi-regional phase II clinical trial for the combination treatment of GT90001 and Nivolumab for the second-line treatment of HCC.
The first patient with advanced or refractory solid tumors in a phase Ib/II clinical trial of GT90001 in combination with KN046, a PD-L1/CTLA-4 bispecific antibody developed by Alphamab Oncology, expects to be dosed soon. Tumor types being targeted include HCC, gastric carcinoma (GC), gastroesophageal junction adenocarcinoma (GEJ), urothelial carcinoma (UC), and esophageal square cell carcinoma (ESCC).
In addition, Kintor Pharma has entered into a partnership agreement with US-based Gensun for its PD-L1/TGF-beta dual-targeting antibody. Kintor Pharma’s clinical trial application for PD-L1/TGF-beta was recently accepted by the China Center for Drug Evaluation (CDE).
Meanwhile, Kintor Pharma is working on designing clinical trials for combination therapy of ALK-1/VEGF dual-targeting antibody for solid tumor types.
Continuous Expansion of Diversified Products Pipeline
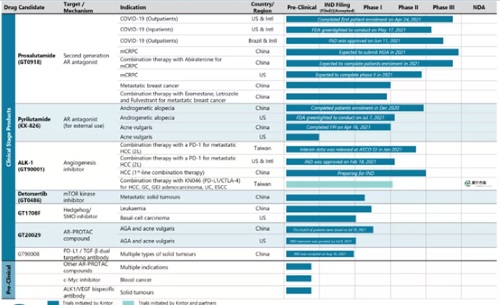
Kintor Pharma’s products pipeline consists of innovative, small-molecule drugs, biologics, and combination therapies. At present, seven of Kintor Pharma’s new drug programs are in clinical development:
— Two AR antagonists (proxalutamide and pyrilutamide)
— ALK-1 monoclonal antibody
— mTOR kinase targeting inhibitor
— Hedgehog/SMO inhibitor
— AR-PROTAC compounds, and
— PD-L1/TGF-beta dual-targeting antibody
Kintor Pharma’s other products – c-Myc inhibitors and ALK-1/VEGF dual-targeting antibody, among others – are under pre-clinical study.
Collaboration with Reputable Partners Globally
Kintor Pharma has partnered with the Medical College of the University of Michigan and the Beijing Proteome Research Center on pre-clinical research to study further the proxalutamide’s mechanism of action against the SARS-CoV-2 virus causing the COVID-19 pandemic. In addition to the three phase III MRCT of proxalutamide for COVID-19, Kintor Pharma has been working with several international CROs to advance clinical progress.
We have expanded production capacity at our own GMP factory and entered into a strategic partnership with Hainan Visum Pharmaceutical Limited to expand the production capacity of proxalutamide. At the same time, we entered into a licensing agreement with Shanghai Fosun Pharmaceutical Development Ltd. (“Fosun Pharma Development”) on the commercialization of proxalutamide for the treatment of COVID-19 infection in India and 28 African countries. In addition, Kintor Pharma has also partnered with PT Etana to commercialize proxalutamide to treat COVID-19 infection in Indonesia. We believe these efforts will make a valuable contribution in the global fight against the COVID-19 pandemic.
Robust Performance in Capital Market
On June 2, 2021, Kintor Pharma completed the top-up placement and raised HK$1.16 billion($150 million) from reputable long-only funds, healthcare specialist funds and hedge funds.
On August 20, 2021, Hang Seng Indexes Company Limited announced the Hang Seng Composite Index (HSCI) results as of June 30, 2021. Kintor Pharma was included in the HSCI, with relevant changes taking effect on September 6, 2021. Being included in the HSCI means that Kintor Pharma’s stock is eligible for inclusion in the Hong Kong Stock Connect program, which will help increase the stock’s liquidity and expand investors base.
2021 Interim Financial Performance
For the six months ended June 30,2021, the company’s R&D costs increased by 90.2% to RMB282.2 million, mainly attributable to the increase in clinical research expenses primarily paid to hospitals and CROs for the clinical trials advancing proxalutamide for the treatment of COVID-19 infection, and the increase in R&D employee expenses primarily due to the expansion of our R&D personnel and the grant of RSUs to certain of our R&D employees under the Employee Incentive Scheme.
Our adjusted loss after adding back the listing expenses (which is applicable to the six months ended 30 June 2020 only) and share-based compensation expenses for the Employee Incentive Scheme increased by RMB136.2 million or approximately 83.2% from RMB163.7 million for the six months ended 30 June 2020 to RMB299.9 million for the six months ended 30 June 2021.
The company’s cash and cash equivalents consisted of deposits (including time deposit) with banks and cash on hand. As of June 30, 2021, cash and cash equivalents (including time deposit) increased from RMB1,388.9 million on December 31, 2020 to RMB1,755.3 million. The increase was primarily attributable to the net cash proceeds of approximately HK$1.16 billion Kintor Pharma received from the placement.
As of June 30, 2021, we had utilized bank facilities of RMB137.7 million and unutilized bank facilities of RMB112.3 million.
About Kintor Pharmaceutical Limited
Kintor Pharmaceutical Limited is developing and commercializing a robust pipeline of small molecule and biological drugs for androgen-receptor-related disease areas with unmet medical needs, including COVID-19, prostate, breast and liver cancer, alopecia and acne. For more information, visit www.kintor.com.cn.