Hua Medicine (HKG:2552), a leading innovative drug development company focused on developing novel therapies for the treatment of diabetes, today announced:
- Successfully completes two Phase III 53-week registration trials in over 1,200+ Chinese patients in the midst of the COVID-19 global pandemic on time and with high quality data and results
- Dorzagliatin is safe and well tolerated in both the 24-week and 52-week periods of the DAWN Trial in Chinese T2D patients who have failed glycemic control with maximum daily dose of metformin (1500mg/day)
- Unlike many other oral antidiabetic drugs, dorzagliatin does not increase hypoglycemia incidence rate when used in combination with metformin: overall hypoglycemia incidence (blood glucose level <3 mmol/L) rate during the 52-week period for the DAWN Trial was less than 1%
- At the end of the 24-week period, the primary efficacy endpoint was 1.02% HbA1c reduction for the dorzagliatin-treated group, together with excellent 5.45 mmol/L reduction of post-meal glucose (2h-PPG) from baseline, and are statistically significant over placebo with p-value less than 0.0001
- HbA1c reduction is potent, fast acting and sustained through the 24-week and 52-week trial periods for patients treated with dorzagliatin
- Similar to observations made in the SEED Trial, conducted with drug-naive T2D patients with average disease history of one year, a significant increase in HOMA2-beta and reduction in HOMA2-IR over placebo were observed in the DAWN Trial, suggesting a consistent improvement of beta-cell function and reduction in insulin resistance in diabetes patients with average disease history of almost six years and who had failed glycemic control on maximum daily dose of metformin (1500mg/day)
- First incidence of a China-based biotechnology company introducing a global first-in-class oral therapeutic – a glucose sensitizer, with a novel mechanism of action that targets the underlying cause of Type 2 diabetes, e.g., insulin resistance and beta-cell function
Type 2 diabetes is a worldwide epidemic fueled by the increasing prevalence of obesity, sedentary lifestyles and poor nutrition. Diabetes is characterized by hyperglycemia, which chronic sustained exposure to is associated with long-term damage, dysfunction, and failure of various organs leading to microvascular complications (e.g., retinopathy, nephropathy and neuropathy), as well as macrovascular complications (e.g., stroke, myocardial infarction and peripheral arterial disease). As a result, diabetes is an expensive disease leading to progressively higher medical costs. In a Consensus Report, Diabetes Self-management Education and Support in Adults with Type 2 Diabetes, published in Diabetes Care in July 2020, the American Diabetes Association, et. al., concludes, “Confounding the diabetes epidemic and high costs, therapeutic targets are not being met. There is a lack of improvement in reaching clinical targets since 2005 despite advancements in medication and technology treatment modalities. Indeed, between 2010 and 2016 improved outcomes stalled or reversed.” The fundamental defect in current diabetes pharmacologic treatment is the failure of current therapeutics to target repair of the underlying cause of Type 2 diabetes (“T2D”) – insulin resistance and beta cell function degeneration. Targeting the repair of the body’s glucose sensor, glucokinase, offers the potential to restore the body’s natural ability to maintain glucose homeostasis. As a first-in-class oral glucokinase activator, dorzagliatin’s mechanism of action is specifically targeting the repair of glucokinase, and extensive clinical data has demonstrated dorzagliatin’s ability to maintain glycemic control, as evidenced by significantly reduced HbA1c levels and post-meal glucose levels (2h-PPG), while exhibiting less than one percent incidence of hypoglycemia (blood glucose level <3 mmol/L) in its two 1,200+ patient Phase III trials in China.
DAWN (also known as HMM0302), the second Phase III trial with dorzagliatin (“The DAWN Trial”) is a 53-week trial (52-week on treatment, plus one-week follow-up), designed to investigate the efficacy and safety of 75mg BID dorzagliatin in 767 T2D patients treated with metformin, with an initial 24-week double blinded, placebo-controlled treatment, followed by an open-label 28-week treatment. The primary efficacy and safety endpoints were evaluated at 24 weeks. The primary objective of the subsequent 28-week trial period was to evaluate and observe the safety profile of dorzagliatin. In both the 24-week double blinded period and the 28-week open-label treatment period, dorzagliatin exhibited a safe and well-tolerated clinical profile. The incidence of adverse events was similar between the dorzagliatin-treated and placebo groups. There was less than 1% hypoglycemia (blood glucose < 3 mmol/L) during the 52-week treatment period. During both the 24-week double blinded period and the 28-week open-label treatment, patients also saw a continued reduction in insulin resistance, as measured by HOMA2-IR (insulin resistance is the hallmark of Type 2 diabetes).
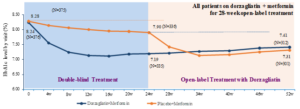
In July 2020, Hua Medicine announced the DAWN Trial had achieved its primary efficacy and safety endpoints over the initial 24-week double blinded period. For the 52-week treatment period, the efficacy and safety profiles were sustained based on the topline data analysis. During the 28-week open-label period, patients initially receiving placebo + metformin (i.e., the placebo group) switched to receive dorzagliatin + metformin. The graph below illustrates the fast onset and sustained efficacy (as measured by HbA1clevels) for the two-cohort groups for the entire 52-week period.
The efficacy and safety results of dorzagliatin in the DAWN Trial mirrored the previously published results from the similarly designed 52-week results of SEED (also known as HMM0301), the first Phase III trial with dorzagliatin (the “SEED Trial”), despite the fact that the SEED Trial enrolled drug-naive T2D patients, with average disease history of one year, whereas the DAWN Trial enrolled T2D patients with average disease history of almost six years and that could not maintain glucose control while on the maximum daily dose of metformin (1500mg/day). Both of the DAWN Trial and SEED Trial have demonstrated that T2D patients administered with dorzagliatin experienced improved beta-cell function, reduced insulin resistance, and substantial post-meal glucose reduction as measured by 2h-PPG levels.
“The results of DAWN have been incredibly positive and recapitulate in patients on baseline metformin therapy the results of the prior large Phase III SEED Trial, in which dorzagliatin monotherapy resulted in a significant decrease in HbA1c that was sustained throughout the 52-week study. DAWN was completed during the COVID-19 pandemic with high quality data and results,” said Dr. Wenying Yang of the China-Japan Friendship Hospital and lead principal investigator of DAWN. “The results indicate very good and fast onset HbA1c reduction with sustained efficacy, improved beta-cell function, and reduced insulin resistance. The study shows that dorzagliatin is safe and well-tolerated over the entire 52-week treatment period with low incidence of hypoglycemia, offering a new solution to T2D patients for whom metformin is no longer sufficient to control blood glucose. Additional Phase I studies conducted with dorzagliatin in combination with DPP-4 inhibitors or SGLT-2 inhibitors in the United States have demonstrated synergies in blood glucose control, suggesting dorzagliatin has a broader potential use in T2D patients with different needs in glycemic control and at different stages of the disease progression.”
“On behalf of the Chinese Diabetes Society, and the Dorzagliatin SEED clinical trial team, we express our heart-felt congratulations to Dr. Yang for leading the DAWN trial and to the Hua Medicine team, especially in successfully completing the trial on time and in high quality during the COVID-19 global outbreak,” said Dr. Zhu Dalong, President of the Chinese Diabetes Society. “We are excited that China has the first opportunity to showcase to the world the benefits of a new first-in-class discovery for Type 2 diabetes, dorzagliatin. In particular, we are excited that dorzagliatin, as a monotherapy, has also demonstrated its potential in a Phase I study with end stage renal disease patients, and therefore, could provide a unique opportunity for diabetes kidney disease patients, who make up 20%-40% of Type 2 diabetes patients globally.”
“On behalf of the Scientific Advisory Board of Hua Medicine, I would like to convey our congratulations to Hua Medicine on successfully bringing this global breakthrough innovation for the advancement of Type 2 diabetes treatment to the world,” said Dr. Bennett M. Shapiro, member of the Hua Medicine Portfolio Advisory Board since September 2010. “Over the last 10 years, we have advised Hua Medicine on its clinical programs for dorzagliatin, and we are excited to see that dorzagliatin has now successfully completed its two Phase III registration trials in over 1,200 patients in China – demonstrating its safety and efficacy in patients with Type 2 diabetes. We now look forward to working with Hua Medicine to provide our guidance and advice on bringing this drug to Type 2 diabetes patients globally, and on further exploring dorzagliatin’s unique mechanism of action for the treatment of diabetes specifically, and on human metabolism in general.” (For more information on the composition of the Hua Medicine Portfolio Advisory Board, and the biography of each member, please refer to https://www.huamedicine.com/En/portfolioadvisoryboard.)
“This has been an incredible 10-year journey for the Hua Medicine team, Chinese investigators, Hua’s partners and supporters. With the successful completion of dorzagliatin’s two registration trials, we have validated Dr. Franz Matschinsky’s half century of work on glucokinase’s central role in glucose homeostasis, bringing to life a new hope of a treatment that addresses the root cause of Type 2 diabetes to the millions of diabetes patients worldwide. We are incredibly proud that Hua Medicine is at the forefront of this global breakthrough by introducing a new therapeutic class of Type 2 diabetes medication – the glucose sensitizer class – that should be able to dramatically advance Type 2 diabetes standard of care to a new level,” said Dr. Li Chen, CEO and founder of Hua Medicine. “In exploring the unique advantages of this new therapeutic class for Type 2 diabetes, we are excited about two investigator initiated trials currently ongoing to support dorzagliatin’s role as a glucose sensitizer. One of the trials studies the effects of dorzagliatin on 1st phase insulin secretion, and another is conducted by select principal investigators from the SEED trial, to study the lasting effects of dorzagliatin, including the clinical remission rate, among other relevant biomarkers, of patients after completion of SEED. We look forward to sharing the results of these landmark studies later in 2021, when our respective investigators are expected to make the results available to us. Hua Medicine is now working as expeditiously as practicable to make our NDA submission, and continue to collaborate with our partner in mainland China, Bayer AG, to prepare for the commercialization of dorzagliatin.”
DAWN Trial study design
DAWN is a randomized, double-blind, placebo-controlled Phase III study in 767 Type 2 diabetes patients whose blood glucose cannot be controlled with the maximum tolerated dose of equal or greater than 1500 mg/day of metformin. Subjects were treated with metformin (Glucophage) at 1500mg/day as basic therapy throughout the whole 52-week treatment period. Patients were given twice-daily doses of dorzagliatin (75mg) or placebo, randomized on a 1:1 ratio. The clinical study evaluated the efficacy and safety of dorzagliatin during 24 weeks of double-blinded treatment, followed by a subsequent 28-week open-label treatment period receiving dorzagliatin 75mg twice daily. The primary efficacy endpoint was evaluated at the conclusion of the first 24 weeks. The trial was conducted in 72 clinical sites across China led by Professor Wenying Yang at China-Japan Friendship Hospital. (NCT03141073).
SEED Trial study design
SEED is a randomized, double-blind, placebo-controlled Phase III study in 463 drug naive T2D patients. Patients were treated with twice-daily doses of dorzagliatin (75 mg) or placebo, randomized 2:1. The clinical study evaluated the efficacy and safety of dorzagliatin during 24 weeks of double-blinded treatment, followed by a subsequent 28-week open-label treatment period, for a total of 52 weeks plus one-week follow-up. During the 28-week open-label period, both patient groups were treated with twice-daily doses of dorzagliatin (75 mg). The trial was conducted in compliance with the guidelines of the Chinese Society of Endocrinology, which require physicians to educate patients and strictly enforce improved exercise and dietary control, as well as continuous self-monitoring, in treating Type 2 diabetes. The trial was conducted at 40 clinical sites across China led by Professor Dalong Zhu, President of the Chinese Diabetes Society. (NCT03173391).
About Dorzagliatin
Dorzagliatin is an investigational first-in-class, dual-acting glucokinase activator, designed to control the progressive, degenerative nature of diabetes by restoring glucose homeostasis in patients with Type 2 diabetes. By addressing the defect of the glucose sensor function of glucokinase, dorzagliatin has the potential to restore the impaired glucose homeostasis state of patients with Type 2 diabetes and serve as a first-line standard-of-care therapy for the treatment of the disease, or as a cornerstone therapy when taken in combination with currently approved anti-diabetes drugs. Two Phase III registration trials for dorzagliatin have been completed in China. The Company has obtained the “Drug manufacturing permit” of dorzagliatin issued by the Shanghai Drug Administration, and plans to submit its NDA to the National Medical Products Administration, so as to realize the “First in Global, Start from China” for the benefit of diabetic patients worldwide.
About Hua Medicine
Hua Medicine is a leading, clinical-stage innovative drug development company in China focused on developing novel therapies for the treatment of diabetes. Founded by an experienced group of entrepreneurs and international investment firms, Hua Medicine advanced a first-in-class oral drug for the treatment of T2DM into NDA-enabling stage and it has successfully completed two Phase III registration trials in China for dorzagliatin. The Company has initiated product life-cycle management studies of this novel diabetes therapy and advanced its use in personalized diabetes care. Hua Medicine is working closely with disease experts and regulatory agencies in China and across the world to advance diabetes care solutions for patients worldwide.
About Hua Medicine Portfolio Advisory Board
The Hua Medicine Portfolio Advisory Board (“PAB”) is comprised of distinguished scientists and corporate management with extensive experience in the biopharmaceutical sector. First established in 2010, the current PAB membership has been comprised of the following scientists since 2012: John J. Baldwin, Ph.D, James S. MacDonald, Ph.D, Bennett M. Shapiro, M.D., Catherine D. Strader, Ph.D, and Chris T. Walsh, Ph.D. Additional information on each of the Hua Medicine PAB members can be found at https://www.huamedicine.com/En/portfolioadvisoryboard.
For more information
Hua Medicine Website: www.huamedicine.com
Investors Email: ir@huamedicine.com
Media Email: pr@huamedicine.com
Issued by Porda Havas International Finance Communications Group for and on behalf of Hua Medicine. For further information, please contact:
Mr. Bunny Lee +852 3150 6707 bunny.lee@pordahavas.com
Ms. Louisa Chen +86 75523807432 louisa.chen@pordahavas.com
Ms. Karen Chiu +852 3150 6726 karen.chiu@pordahavas.com
Ms. Winnie Tan +852 15915975512 winnie.tan@pordahavas.com